Abstract
Introduction
Current front-line induction therapies result in a median progression-free survival (PFS) of 2.6-6.4 years in patients with follicular lymphoma (FL). However, survival outcomes may vary based on prognostic factors at diagnosis. The FL International Prognostic Index (FLIPI) identifies 3 risk categories based on pre-treatment clinical and laboratory features, and this index predicts survival based on risk score. However, it was developed primarily before the use of rituximab. We evaluated the utility of FLIPI in the current era at our institution.
Methods
All FL patients with available baseline, treatment, and follow-up data were included. We assessed each patient for FLIPI risk score (risk category and actual score), treatment response, and incidence of first relapse. We evaluated baseline characteristics of interest and estimated PFS and overall survival (OS) based on the Kaplan-Meier method. PFS was defined as the time from the date of diagnosis to the date of relapse, death without relapse, or last known follow-up. OS was defined as the time from the date of diagnosis to the date of death or last known follow-up. We used a Fisher's exact test to determine the association of FLIPI risk with other baseline features. We utilized the log-rank test and Cox proportional hazards models to compare survival outcomes between the low-, intermediate-, and high-risk FLIPI groups.
Results
Of 132 patients, median age at diagnosis was 58 years (22-83), and 71 (54%) were female. Most patients (n=107, 81%) had grade 1-2 disease, and 100 patients (76%) had stage 3-4 disease.
A FLIPI score of 0 was found in 10 patients (8%), of 1 in 20 (15%), of 2 in 41 (31%), of 3 in 43 (33%), of 4 in 10 (8%), and of 5 in 3 patients (2%). An additional 5 patients had incomplete data but had at least a score of 3 based on available data and were therefore considered high-risk. As a result, 30 patients (23%) were categorized as low-, 41 (31%) were categorized as intermediate-, and 61 (46%) were categorized as high-risk. Median time from diagnosis to treatment initiation was 3 months (0.85-49) for low-, 2 months (0.2-115.6) for intermediate-, and 1.6 months (0.3-132.3) for high-risk patients. R-CHOP was given to 41 patients (31%), single agent rituximab to 22(16%), BR to 16 (12%), VR-CHOP to 16 (12%), R-CVP to 9 (7%), and other conventional therapies to 28 patients (21%). Seventy-five (57%) patients received maintenance therapy.
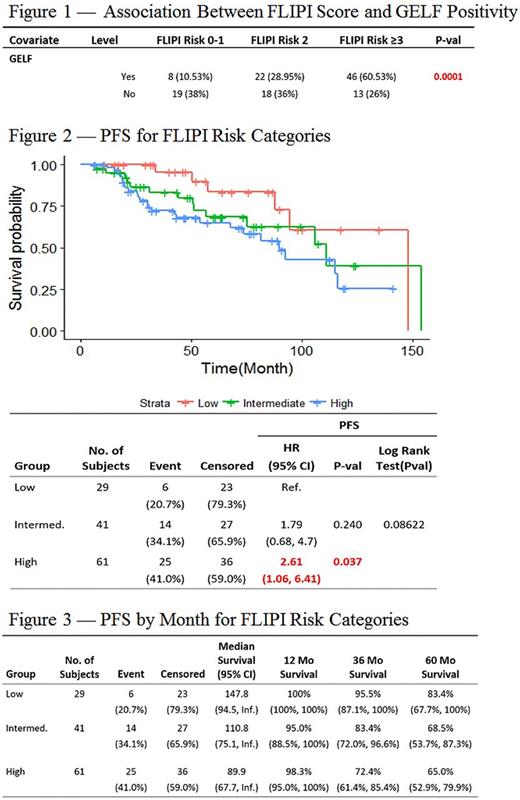
Patients were observed for a median of 63 months. Those with high-risk FLIPI scores were more likely to fulfill at least one GELF criterion (n=46; 75% of high-risk patients), relative to those with low-risk FLIPI scores (n=8; 27% of low-risk patients; p= 0.0001; Figure 1). Six (20%) of the 30 patients with a low-risk FLIPI score relapsed during the observation period as did 14 (34%) of those with an intermediate- and 25 (41%) of those with a high-risk score. The risk of relapse was significantly greater in those with high-risk FLIPI relative to those with low-risk scores (RR= 2.05 [1.03, 5.04]). High-risk patients also had a significantly shorter median PFS (44 months) relative to that of low-risk patients (57 months; HR= 2.61[1.06, 6.41], p=0.037; Figure 2). Three year PFS for low-, intermediate-, and high-risk groups were 95.5% (87.1%, 100%), 83.4% (72.0%, 96.6%), and 72.4% (61.4%, 85.4%) respectively (Figure 3). However, there was no significant difference in OS between the high-risk and low-risk groups (HR= 2.37[0.48, 11.58], p= 0.288). Three-year OS for low-, intermediate- and high-risk groups were 100%, 100%, and 96.1% (91%, 100%) respectively. No significant differences in outcomes were found between the intermediate and low- or high-risk groups.
Conclusion
In the rituximab era, the FLIPI score at diagnosis continues to have significant prognostic value with respect to PFS. Those with a FLIPI ≥3 have a higher risk of relapse and relapse significantly earlier relative to those with a FLIPI score of 0-1. However, no significant difference in OS was found between patients in different FLIPI risk categories. Predicting early relapse in FL remains important due to the poor survival outcomes that exist for this population (Casulo et al JCO 2015). Given the high risk of patients experiencing early relapse in FL, identification of at-risk patients may allow for consideration of alternative therapies to avoid early relapse. Larger studies across multiple centers are recommended to further confirm this conclusion in the rituximab era.
Calzada: Seattle Genetics: Research Funding. Flowers: TG Therapeutics: Research Funding; Bayer: Consultancy; Eastern Cooperative Oncology Group: Research Funding; Millennium/Takeda: Research Funding; Clinical Care Options: Research Funding; OptumRx: Consultancy; Pharmacyclics LLC, an AbbVie Company: Research Funding; Research to Practice: Research Funding; Educational Concepts: Research Funding; Prime Oncology: Research Funding; Celgene: Consultancy, Research Funding; Onyx: Research Funding; Gilead: Consultancy; Genentech/Roche: Consultancy, Research Funding; V Foundation: Research Funding; Infinity: Research Funding; National Cancer Institute: Research Funding; Spectrum: Consultancy; Janssen Pharmaceutical: Research Funding; Seattle Genetics: Consultancy; Acerta: Research Funding; National Institutes Of Health: Research Funding; Burroughs Welcome Fund: Research Funding; Abbvie: Consultancy, Research Funding. Cohen: LAM Therapeutics, Inc: Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takada: Research Funding; Bristol Myers Squibb: Research Funding; Bioinvent: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Infinity: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.